May 22, 2025 | Digital Dermatopathology, Digital Pathology, Pathology Business
The 2025 Ogden Surgical Medical Society Conference hosted medical thought leaders from across specialties. What was the burning question on the mind’s of many health professionals? How is artificial intelligence reshaping healthcare—from diagnostics to documentation—and what it means for providers navigating population health and value-based care models?
Greg Osmond, MD, MPH, co-founder of PathologyWatch, and a presenter at this year’s event, positioned the answer as a strategic balance of Pathology, AI, and Population Health.
Here’s a look at the highlights and key takeaways from his session:
Finding the Balance: Pathology, AI, and Population Health
Dr. Osmond opened by framing the most current questions facing clinicians and healthcare organizations today:
- What does an effective balance of pathology, AI, and population health actually look like?
- What can today’s AI really do in clinical practice?
- How can clinics and providers begin to build realistic, AI-enabled workflows?
- And where are we already seeing AI deliver value?
AI in Action: Real-World Applications
Throughout the session, Dr. Osmond highlighted real examples of AI tools currently making waves in healthcare:
- Clinical Documentation: Tools like Nuance DAX Copilot, Microsoft Azure, and Suki.AI help automate note-taking and improve physician efficiency.
- Patient Communication: AI is now generating email responses via EPIC and DAX Copilot, lightening the admin load for busy clinicians.
- Radiology and Diagnostics: Platforms like Viz.AI assist with triage and interpretation in high-volume imaging environments.
- Cardiology: Tools like Cleerly are enabling more precise characterization of coronary artery disease, while Caption Health (GE) supports AI-guided echocardiograms by non-specialists.
- Pathology Diagnostics: Companies like PathAI, PaigeAI, PathologyWatch, and Proscia are transforming the pathology workflow.
- Genomics: Precision medicine platforms such as Tempus are bridging AI with genetic insights to personalize treatment.
Key Insights from Dr. Osmond’s Talk
1. Clinical Context Still Matters
One of the most practical takeaways? Always tell your pathologist what you think you’re sampling—even if you’re unsure. That clinical context shapes how the specimen is interpreted.
“It doesn’t matter if you’re right or wrong—it gives us context to help,” Dr. Osmond said.
For example, distinguishing between lichenoid keratosis and lichenoid interface dermatitis is largely a result of clinical context.
2. Frontline Providers Must Lead the AI Transition
As AI adoption accelerates, Dr. Osmond stressed the importance of input from practicing clinicians. Providers are best positioned to identify workflow friction, operational inefficiencies, and areas where automation makes a real difference.
“AI doesn’t need to be flashy—it needs to solve the right problem and fit into a feasible business model,” he noted.
In short: Those closest to the clinical work need to shape the tools, not just use them.
3. Population Health Needs Real-Time, Actionable Data
From wearable devices to remote patient monitoring, the potential for AI in population health is huge. But to make it work, organizations must link comprehensive clinical data with real-time inputs.
Dr. Osmond challenged the audience to think critically about incentives in value-based care, asking:
- What is the most actionable data?
- What operational model can turn that data into better outcomes?
- How do we build systems that are financially and logistically feasible?
AI in Healthcare: A Moral and Strategic Imperative
The talk ended with a provocative question: What is our obligation when AI or agentic workflows are objectively better—and already available?
The healthcare AI revolution isn’t on the horizon. It’s here. And according to Dr. Osmond, it’s up to providers and healthcare leaders to guide its ethical, effective, and scalable implementation.
“Physicians and healthcare organizations hold the key data. We also hold the responsibility to use it wisely.”
Final Thoughts
As the healthcare industry continues its shift toward proactive, data-driven, value-based models, the integration of AI into healthcare, especially through the lens of population health, isn’t just inevitable—it’s essential.
Dr. Osmond’s insights remind us that while the tools are evolving fast, the mission remains the same: provide high-quality care, reduce friction, and improve outcomes for the communities we serve.
Apr 24, 2023 | Dermatology Practice, Digital Dermatopathology, Pathology Business
By April Larson, MD
Thirteen is shaping up to be a very fortunate number for digital pathologists in 2023. Thanks largely to the efforts of the College of American Pathologists (CAP), the American Medical Association CPT Editorial Board developed 13 new Category III digital pathology digitization procedure codes. The 13 new add-on CPT codes, which have been introduced to record the use of digital pathology, went into effect on January 1.
Prior to 2023, lab reports in the US used the same CPT (current procedural terminology) codes in reporting any diagnostic read—with no distinction made as to whether the diagnosis utilized digital pathology or a glass slide under a microscope. Thus, both procedures earned the same reimbursement rates.
The new CPT codes will help track the additional work and investment of digital pathology into practice and help establish a new standard of care by demonstrating its wider acceptance and usage by the medical community, which in turn is a big step in receiving reimbursement for those services. This will continue to push the medical industry toward the adoption of digital pathology, increasing the availability of remote pathology work for pathologists.
Let’s look at the difference between Category I and the new Category III CPT codes and how they could lead to reimbursement rates for those practices utilizing digital pathology.
The Difference Between CPT Codes
The 13 new Category III codes are designed to be temporary in nature. They’re intended for emerging technology, services, and procedures and allow for the data collection directly associated with carrying them out. The goal is to show that these procedures are becoming more commonly adopted so that pathologists can then work with the AMA to shift these codes to Category I status.
I believe the use of these new CPT codes is a helpful measure that the government can use to determine whether new technology—in this case, digital pathology and the use of AI prognostics— is actually advancing the standard of care.
How do Category III codes differ from Category I codes? According to CAP, the new Category III codes may not meet one or more of the following Category I requirements:
- All devices and drugs necessary for the performance of the procedure or service have received FDA clearance or approval when such is required for the performance of the procedure or service.
- The procedure or service is performed by many physicians or other qualified health care professionals across the United States.
- The procedure or service is performed with a frequency consistent with the intended clinical use (e.g., a service for a common condition should have high volume, whereas a service commonly performed for a rare condition may have low volume).
- The procedure or service is consistent with current medical practice.
- The clinical efficacy of the procedure or service is documented in literature that meets the requirements set forth in the CPT code change application.
Category III codes should be reported only for primary diagnostic use; they should not be reported if the digitization performed is solely for archival or educational purposes, developing a database for training or validation of AI algorithms, or for conference presentations.
The 13 new codes are attached to different services and procedures, but the one thing they all have in common is involving the digitization of glass slides.
The use of these codes is exciting both for dermatologists and dermatopathologists. What we’ve seen at PathologyWatch is that dermatopathologists can benefit from remote digital workflows, and dermatologists have quicker access to both digital slides and reports.
Reclassification to Category I codes, which is the goal, requires meeting both general and specific criteria as determined by the AMA.
Potential Game-Changer for Pathologists
While temporary in nature, the 13 new codes have the potential to be revolutionary for digital pathologists for a variety of reasons. Of primary merit is that the codes are widely expected to achieve Category I status in the near future, opening the door to new financial reimbursements.
Clearly, there are significant upfront expenses associated with digital pathology. The initial technology investment, for example, can seem formidable, with scanners running anywhere from $250,000 to $1 million.
While it is important to note that there are presently no reimbursements directly tied to the new CPT III codes, the change is laying the groundwork by bringing a different dynamic into play.
The utilization of CPT codes helps establish the frequency of usage within the medical community. In order to determine reimbursement, this is often determined by committees of experts who help document the financial investment required to use a new technology.
Much like radiology, the wide adoption of digital pathology will help improve the quality of patient care by promoting sharing of information and images with consulting providers, which improves communication and coordination of care. It also promotes more frequent peer-to-peer and expert consultation with difficult cases and patient education and understanding of their disease.
Reimbursement also provides a financial incentive for clinics and labs to invest further in digital pathology. CAP proposals are being considered for development in the next few years through the AMA CPT process. In the meantime, it is important for dermatologists and dermatopathologists to use the new Category III codes to properly track their digital pathology services.
View a chart with the new CPT codes and detailed explanations of what they entail at cap.org. Then, contact us to learn more about how these new codes, and the adoption of digital pathology, could greatly improve your level of patient care and your practice in general.
— April Larson, MD, is chief medical officer and a cofounder at PathologyWatch.
Mar 27, 2023 | Digital Dermatopathology
PathologyWatch, the groundbreaking leader of digital dermatopathology services, has been chosen as one of a select group of 23 startups to participate in the global AWS Healthcare Accelerator: Global Cohort for Workforce.
This AWS Healthcare Accelerator is a four-week technical, business, and mentorship program for startups seeking to use AWS to improve healthcare workforce training, retention, and deployment. This opportunity, which begins in April and runs through the summer, will support PathologyWatch’s efforts to digitally optimize pathology workflows, providing tools that augment dermatopathology resources and interface directly with EHRs. PathologyWatch’s academic-level dermatopathologists can review and provide interpretations for digital slides remotely, supporting clinical decision-making while reducing costs and allowing workplace flexibility.
“We are honored to be one of a few companies chosen for the AWS Healthcare Accelerator program,” says Dan Lambert, CEO and cofounder of PathologyWatch. “Digital pathology is the key to providing quality healthcare solutions to so many underserved areas of the world, including the rural United States. Our mission at PathologyWatch is to leapfrog technology forward in the digital pathology space by connecting individual offices with dermpath experts throughout the world. We believe the AWS Accelerator program can help us in that journey.”
Curriculum of the AWS Healthcare Accelerator will offer opportunities such as hands-on AWS Cloud and technical training, mentorship from healthcare leaders, and exposure to AWS customers and members of the AWS Partner Network. PathologyWatch, which is seeking to advance solutions for addressing urgent challenges facing the healthcare workforce, will also receive AWS computing credits and opportunities to speak with investors and industry experts, including at a Demo Day, where PathologyWatch’s solutions will be showcased.
“Solutions to help clinicians as well as other office and technical staff in healthcare are needed urgently and globally,” says Dr. Rowland Illing, chief medical officer and director of International Public Sector Health at AWS. “We know that advancing cloud- and technology-enabled approaches can alleviate some of the burden, and we’re proud to be convening standout startups and healthcare leaders in this first-ever global Accelerator to do that.”
“We do a significant amount of AI and deep learning research on AWS. They’ve been a great partner,” says Lambert. “The sample volume that we process daily has gone up almost 200% over the past couple of years, and AWS has been able to scale with us. It’s nice having a flexible on-demand hosting solution available immediately.”
Healthcare workforce shortages are at crisis levels, driven by burnout, shrinking budgets, and the aftermath of a worldwide pandemic. A shortfall of 10 million healthcare workers is forecast by 2030. As a result, patients may go untreated or experience delays in care, and healthcare workers need support now more than ever.
In the field of pathology, there is a significant shortage of qualified pathologists, both in the US and worldwide. PathologyWatch is using digital tools to help cover this physician gap, allow pathologists to work remotely, and reduce burnout.
For more information on the AWS Healthcare Accelerator, visit alchemistaccelerator.com/AWS-Healthcare-Accelerator.
Mar 21, 2023 | Digital Dermatopathology, Digital Pathology, Meet The Team, Pathology Business
We had a wonderful time at AAD’s annual conference held last weekend in New Orleans. With 8,500 health care professionals and over 16,000 total registrants, The Big Easy was bustling with the latest in pathology resources and expertise.
Here are a few of the highlights from the PathologyWatch team:
This year’s booth enjoyed a vibrant stream of dermatologists who were interested in learning about dot., the dermpath virtual assistant that helps derms to manage case workloads, view digital images and case files, create detailed pathology reports, and collaborate with colleagues.
Dr. Cacey Peters was on hand to demonstrate how dot. makes reviewing and annotating digital slides a snap for Derms and DermPaths. He also met with more than a few starstruck fans of his popular #GettingUnderYourSkin social media videos.
Though it isn’t polite to brag, we put our best foot forward by producing the show’s most memorable swag: custom socks featuring verruca vulgaris histology. That’s right, we gave warts to hundreds of happy derms.
In addition, a hot pot of Starbucks coffee is just what the doctors ordered to stay alert and keep their eyes on the prize.
Friday evening we hosted our infamous annual AAD happy hour at Rosie’s On The Roof, which is located on top of the historic Higgins Hotel. Hundreds of Derms braved stormy conditions to join us for a classic fais do-do with bottomless hors d’oeuvres and drinks.
Falling on St. Patrick’s Day, the place was packed with festive pathologists looking to let off a little steam and socialize between conference sessions.
The patio’s city views offered the perfect location to reunite with familiar faces and make new friends.
At the end of the night, we gave away special edition “Show Me Some Skin” T-shirts, with the words “Don’t Worry, I’m a Dermatologist” emblazoned on the back. Based on the frenzy they created, the shirts will definitely be returning for AAD in San Diego 2024.
We also handed out custom Mardi Gras beads to get everyone in the spirit of the city’s rich traditions.
Looking back at our experience in New Orleans, we couldn’t be happier to be a part of this inspiring community. It was a joy to meet, answer questions, and share our vision with so many talented Derms.
2023 promises to be a herald year for PathologyWatch, our customers and partners as we continue making great strides in the development of technology and workflows that enhance patient outcomes.
Nov 18, 2022 | Digital Dermatopathology, Meet The Team
A noted dermatopathologist, Dr. Willman joins the PathologyWatch team to continue delivering the highest-quality care to patients.
PathologyWatch, a full-service digital dermatopathology solution, welcomes Dr. Joseph Willman, MD, to its team of respected pathologists. Dr. Willman, a dermatopathologist with over two decades of experience, will utilize state-of-the-art digital pathology workflows to deliver optimal patient care to dermatology clinics.
A board-certified pathologist and dermatopathologist, Dr. Willman completed his residency at the University of Utah, his dermatopathology fellowship at the University of Colorado, and his molecular genetic pathology fellowship at the University of Michigan.
Dr. Willman’s connection to pathology began long before his career and training. His father was a pathologist, introducing him to the profession at a young age. Still, he expected to follow a different career path. As a student, he spent time working in emergency rooms and found surgery and internal medicine interesting. However, his third-year medical school pathology rotation cemented his interest in pathology, and he now sees digital pathology as the future of the field.
“I think the majority of anatomic pathology will be done digitally, as technology advances to digitize the slides, because it makes it so easy to collaborate, get opinions on cases and implement quality measures to get multiple people reviewing a single case,” Dr. Willman said. “It’s so much more convenient than shipping or using a courier to share glass slides, which is easily a 48-hour process by itself. PathologyWatch can secure expert opinions from people all over the country in a single afternoon, and that’s revolutionary.”
With 20 years of experience in dermatopathology, Dr. Willman brings extensive expertise to PathologyWatch. Most recently, he served as the director of a national reference laboratory and molecular diagnostics lab, where he worked extensively with quality systems and compliance and regulation. His experience and interest in lab operations will help as PathologyWatch builds a digital laboratory platform incorporating quality measures that aren’t possible in a physical laboratory.
“We are thrilled to have Dr. Willman joining our team, not just for the years of experience he has but also for his enthusiasm for the future and commitment to progress and innovation,” said Dan Lambert, CEO at PathologyWatch. “He will be of particular help in moving our molecular efforts forward.”
Apr 1, 2022 | Digital Dermatopathology
Hosted in Boston, MA, this year’s Annual AAD convention—held March 25–29, 2022—promised more than a feeling: It marked a roaring return to in-person events for attendees worldwide. It felt good to be back among friends, colleagues, customers, and industry peers.
This year’s event attracted over 13,000 guests to the Boston Convention and Exhibition Center, including 7,000 healthcare professionals. And the PathologyWatch team was there!
Here are some of the highlights:
Friday, March 25 (Convention Day Arrives)
The show opened at 10 a.m., and we enjoyed a steady flow of traffic for most of the day, thanks to our premium location near the main Hall A entrance. Our booth’s design and messaging helped our representatives introduce dermatologists to digital pathology’s advantages.

It was refreshing to reconnect with colleagues and clients across the country. This included a visit from Dr. Roman Bronfenbrener, who stopped by to adore his larger-than-life testimonial banner. He picked it up after posing for photos and paraded it around other booths!


PathologyWatch hosted two events at this year’s AAD convention: First, we hosted a happy hour on the convention’s first night. Second, we cosponsored a private dinner with Castle Biosciences, which provided a unique opportunity to promote our technology, introduce our founders and clinical team, and connect with valued guests and customers before the convention ended.

We selected City Tap, a nearby restaurant and bar, to arrange our happy hour event. The weather was perfect, and the private patio space worked really well.

We set up a central station of food and beverages to encourage people to mingle freely. There was plenty of room for networking, with tables and chairs available for people who wanted to relax and talk after a long day on the convention floor.

Soon, there were over 100 people engaged in dozens of conversations around the outdoor space. Our executive team and sales directors easily mixed in with the crowd, answering questions and bonding over hot wings and pizza.


Later in the evening, strings of outdoor lights brightened up the event and kept discussions going strong. The happy hour was a memorable and impactful part of the convention. We were able to get reacquainted with familiar faces and meet many new people.


Saturday, March 26
We developed the Digital Digest Quiz, a 12-question pathology test featuring digital slides on the PathologyWatch slide viewer to give people a reason to stop by the booth. Placing the video monitor at the front of the booth was visually appealing and created a simple segue to inspire conversations about our proprietary technology.
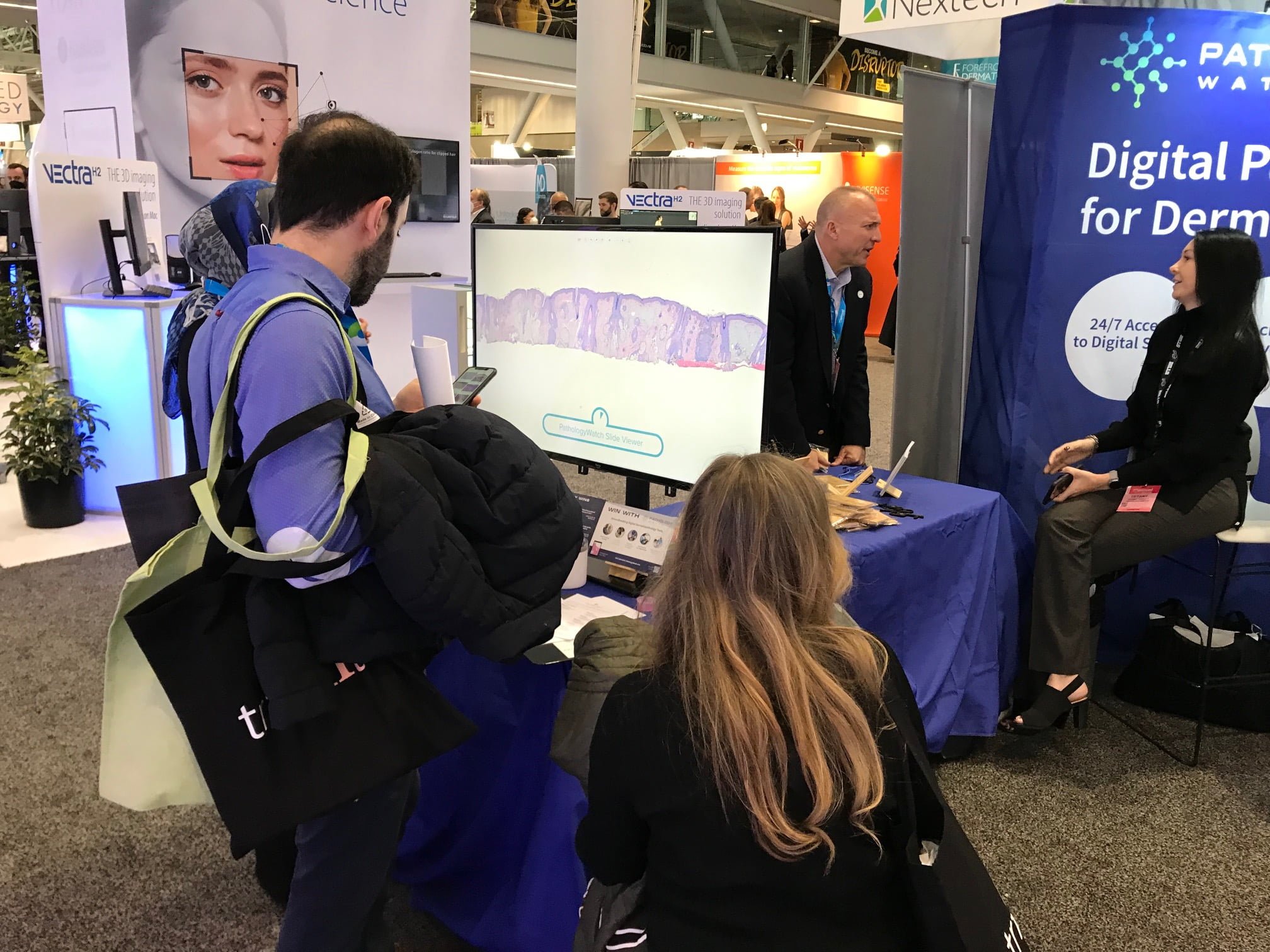
That evening, we gathered at Oceanaire Seafood Room for a cosponsored dinner with Castle Biosciences. We reserved a private room behind the bar that offered the perfect setting for an intimate meal with industry members

Even though it’s only April, it’s been an incredible year for PathologyWatch as we continue to expand our technology and services to support dermatological experts across the country. Thank you to all of our customers and partners who support us in our quest to innovate digital pathology and patient care.





















